Are you involved in recruiting patients for a research project, or are you involved in the initial and follow-up visits of patients participating in clinical studies? We are proud to offer tools to track patients enrolled in research and optimize the collection and analysis of their data.
Omnimed is developing a suite of functionalities to facilitate pre-consent to be contacted for clinical research purposes, and for electronic consent in clinical studies. Within the next few years, electronic medical records will be required to inform clinical studies in which patients participate to increase transparency in the delivery of care, facilitate communication between the care team and clinical study leaders, and simplify the patient's life in becoming a proactive participant in studies that are relevant to their health condition. These requests come from patients, clinicians, and governing bodies alike.
⚙️ Pre-configuration: Adding a program to Omnimed
If you are a clinical study sponsor, a clinician conducting follow-up visits for a clinical study, or a recruitment site, please contact us at info@omnimed.com for assistance in adding your protocol to the Program section of the EMR. If the study you are participating in is not listed, please advise the clinical study leader to contact us to learn more about our services related to the optimization of your processes.
Register a patient to the program
In order for the data to be retrieved and transmitted to the program managers, it is imperative to register the patient through their file in Omnimed.
Here is how to proceed:
- First, log into app.omnimed.com/omnimed (an account must have been created in your name; you should have received your Omnimed ID and password).
- Search the patient record using the search bar in the blue band at the top of the EMR and click on the patient name to open it (learn more about the patient record search). If no patient record exists in Omnimed, you will need to create the patient record.
- Click on the + in the Programs section of the summary (right column) of the patient record.

*Please note that the order of the summary boxes may not be the same as in the screenshot as you can rearrange the summary boxes order. - Select the registration date and the program in which you want to enroll the patient.
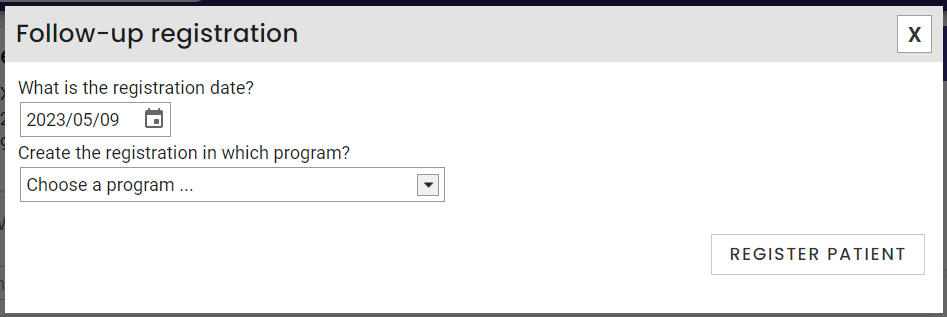
- Click on Print the program registration form to have the patient sign it. Once signed, take a picture of the form and add it to the patient's record. You will then find it in the clinical note of the day.
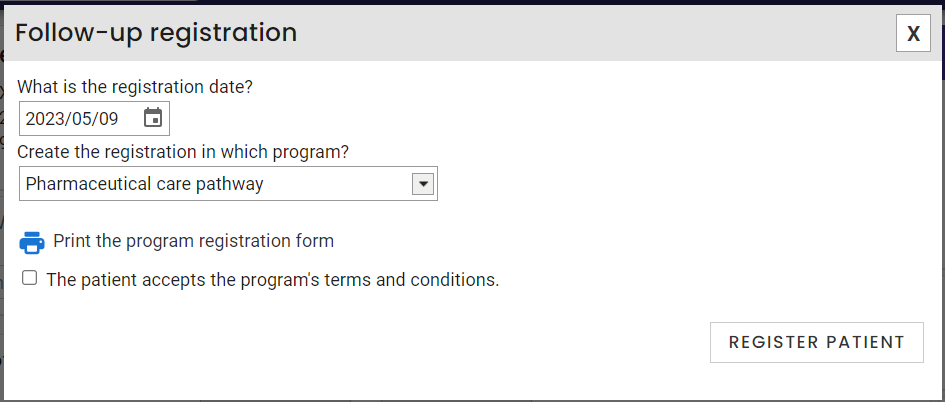
- Check the box The patient accepts the program's terms and conditions.
- Click on the Register patient button.
Once the patient is enrolled in the program, the follow-up can begin.
Document the patient’s visit
Within each program, tools have been developed to facilitate monitoring and standardize data collection.
For each patient visit, here are the steps to follow:
- Prerequisites: Log in to Omnimed, search for the patient record, and make sure the patient is enrolled in the program (see steps above).
- Start a new clinical note by clicking in the Consultation reason field.

- Add a title to the clinical note in the Consultation reason field.
- Then, select the tool to fill in, either by:
- clicking on it directly in your list of clinical tool favorites (drag your cursor over the group to see the complete name of the tool). The complete list of tools related to each program is at the end of this article;

- by searching for it with the help of the search bar or;

- by selecting it from the drop-down menu of the group associated with the clinical tool.

- clicking on it directly in your list of clinical tool favorites (drag your cursor over the group to see the complete name of the tool). The complete list of tools related to each program is at the end of this article;
- Save the note regularly (there is no automatic save in Omnimed clinical tools at this time).

- Complete the note once it is finished (the action to complete the note is equivalent to the signature of the note on paper.
If needed, you can edit an existing entry.
📁 Document a project outside of the medical record
Omnimed can help you in collecting real-world evidence for clinical research purposes. Since some information cannot be recorded in the medical record directly, we have developed a feature to help you in this context: Write a note outside the clinical record (Project Note)